It may share electrons with an adjoining atom to make a covalent bond, or it could take one electron away to kind an ionic bond. As a outcome, halogens are essentially the most reactive nonmetals, as they solely require one electron to type bonds. To create a covalent hyperlink, they either remove an electron from one other atom or share an electron from another storm. Because the valence electrons are at progressively larger energies in groups, the nonmetal’s reactivity reduces as a result of the atoms are unable to gain stability by obtaining electrons. The internal transition metals are the weather within the two rows which are most often shown below the rest of the opposite elements in the periodic desk. It is safest to keep in mind that the inside transition metals have three valence electrons, however as is usually the case, there are a few exceptions to that rule.
Lewis buildings, right here, comes into the picture the place the outer-shell electrons current in an atom are represented as dots. These structures are also referred to as electron dot diagrams. Atom both gain an electron, lose an electron, or share electrons to form a covalent bond to attain an inert gasoline configuration.
How Do Valence Electrons Relate To The Periodic Table?
Here, the electron configuration of iron ion(Fe2+) is 1s2 2s2 2p6 3s2 3p6 3d6. This electron configuration reveals that iron ion(Fe2+) has three shells and the last shell has fourteen electrons. For this, iron ion(Fe2+) has a total of fourteen valence electrons. Again, the iron atom donates two electrons in 4s orbital and an electron in 3d orbital to transform iron ion(Fe3+). The valence electrons decide the properties of the factor and take part within the formation of bonds.
- Determining the precise variety of valence electrons in transition metals includes ideas of quantum concept that are past the scope of this text.
- The electrons current in the outermost orbit are often identified as valence electrons or outer-shell electrons.
- Since CN− ion is a powerful field ligand, it causes the pairing of unpaired 3d electrons.
- The atom with electrical configuration 2, eight, 3 is Aluminium and you can find it in Group three of the periodic table.
Electron Sets are pairs of electrons around the central atom. Earn 10 reputation in order to answer this question. The popularity requirement helps shield this question from spam and non-answer exercise. Connect and share data within a single location that is structured and easy to go looking. That law governs all kinds of phenomena, together with rocket engines, collisions between electrons, and automotive wrecks.
TiCl4 and Mn2O7, then again, are both liquids at room temperature, with melting points beneath 0oC and relatively low boiling points, as may be expected for covalent compounds. They additionally dissolve in water to provide aqueous solutions that conduct electricity, as can be anticipated. Valence electrons This is the currently chosen item.
At some level the columns could presumably be flippantly shaded and a legend could be added. This works greatest when either all the knowledge is on the table or when discussing properties. A cathode ray tube is a glass tube with two electrodes that are connected to an influence valence electrons in iron source supplying electricity. The anode has a small hole in order that the rays can pass by way of. A phosphor coating on the opposite finish of the tube glows when the cathode rays strike it. Gases which do not mix with different components to kind compounds.
What Are The Forms Of Chemical Reactions?
Both valency and valence electrons are applied for any chemical factor. This is a color-coded table made up of many various squares that lists all of the chemical elements recognized to humankind. You can normally discover these inside the quilt of chemistry textbooks. There is also a wonderful interactive table obtainable online here. The periodic table is split into four blocks by electron configuration.
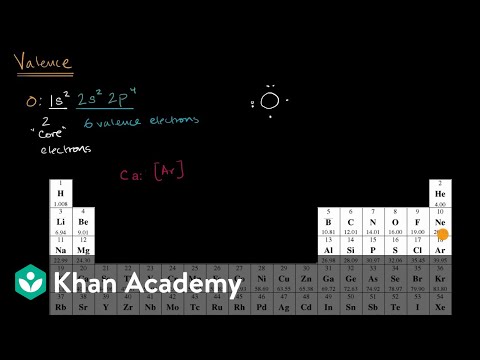
In Electron dot diagrams, a component is represented by its chemical symbol surrounded by dots representing the outer-shell electrons. Another way to find or decide these electrons is through the atomic number. When the difference between the electronegativities of the weather in a compound is relatively massive, the compound is finest categorized as ionic. By overexposing to phosphorus, employees in match factories had been developed a painful and debilitating deformation of jawbone generally known as phossy jaw.
Electrons are distributed in different power shells denoted by (,,,…) generally recognized as the digital configuration of the component. By figuring out the digital configuration of an element, we can decide the number of valence electrons present within the atom’s valence shell. The electrons spend most of their time on the chlorine atom. In this blog we will decide the number of valence electrons in phosphorus atom. This blog will provide you detailed details about phosphorus and it’s valence electrons.
Scandium does not only exist within the +3 oxidation state. The periodic desk is normally given in exams so… Silicon with ninety six.5% – 99.5% purity may be made by decreasing quartzite or sand with extremely purified coke. The discount course of takes place in presence of extra SiO2 which helps to cease the production of silicon carbide . The electromagnetic force between the electrons lets you decide up the cup, counteracting gravity in the course of. And as a substitute of carrying vehicles and vans, it’s going to carry electrons.
Phrases Close By Valence Electrons
She holds teaching certificates in biology and chemistry. Look in the second to final column on the proper hand facet, subsequent to the inert gases. Which one of many following isn’t an allowed configuration? 1s2 2s2 2p6 2d1 1s2 2s2 3s1 1s2 2s2 2p6 3s2 3p5 1s2 2s2 2p6 3s2 3p1 1s2 2s2 2p6 3s Explain why. This is a recorded trial for college kids who missed the final reside session. If you desire a Periodic table with Valence electrons, then go to Periodic table with Valence electrons labeled in it.